Combining Capacity Of Nitrogen . Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). The combining capacity of an atom is known as its valency. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p x 3, therefore it could be expected to make 3. The two nitrogen atoms are bonded together by a triple bond,. The number of bonds that an atom can form as part of a compound is expressed by the. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. Nitrogen does not form stable. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound.
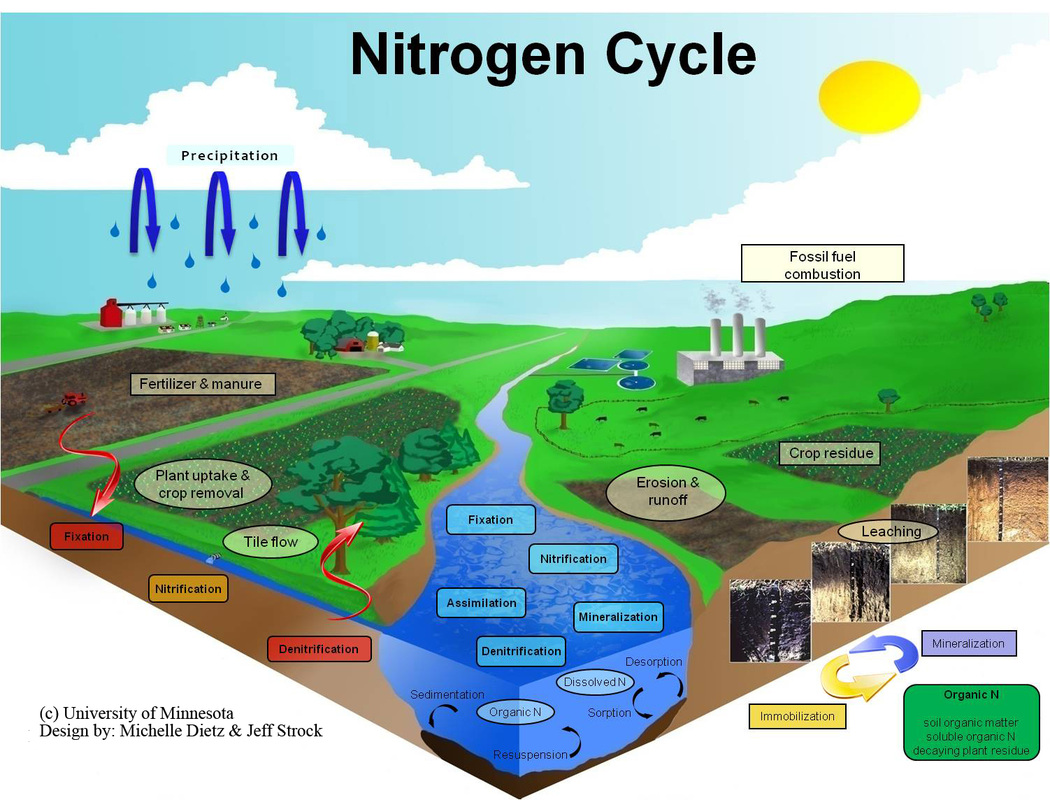
from ecologyconnections.weebly.com
Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. The number of bonds that an atom can form as part of a compound is expressed by the. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). The two nitrogen atoms are bonded together by a triple bond,. The combining capacity of an atom is known as its valency. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p x 3, therefore it could be expected to make 3. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. Nitrogen does not form stable.
Nitrogen Cycle Ecology Connections
Combining Capacity Of Nitrogen An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. Nitrogen does not form stable. The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). The combining capacity of an atom is known as its valency. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The number of bonds that an atom can form as part of a compound is expressed by the. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The two nitrogen atoms are bonded together by a triple bond,. The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p x 3, therefore it could be expected to make 3. Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element.
From www.usgs.gov
Basic overview of the nitrogen cycle U.S. Geological Survey Combining Capacity Of Nitrogen The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). The combining capacity of an atom is known as its valency. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The nitrogen has an electronic configuration 1sx2. Combining Capacity Of Nitrogen.
From blogs.agu.org
Unprecedented levels of nitrogen could pose danger to Earth’s Combining Capacity Of Nitrogen An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The nitrogen has an electronic configuration 1sx2 2sx2 2px3. Combining Capacity Of Nitrogen.
From www.ingridscience.ca
Nitrogen Cycle with molecular modelling ingridscience.ca Combining Capacity Of Nitrogen Nitrogen does not form stable. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). The combining capacity of an atom is known as its valency. The combining capacity of nitrogen, often referred to as its valency, is variable depending on. Combining Capacity Of Nitrogen.
From news.mit.edu
How metals work together to weaken hardy nitrogennitrogen bonds MIT Combining Capacity Of Nitrogen The two nitrogen atoms are bonded together by a triple bond,. The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p x 3, therefore it could be expected to make 3. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The simplest compound of nitrogen is molecular. Combining Capacity Of Nitrogen.
From www.vectorstock.com
Molecular model of nitrogen n2 chemical molecule Vector Image Combining Capacity Of Nitrogen The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. The number of bonds that an atom can form as part of a compound is expressed by the. The simplest compound of. Combining Capacity Of Nitrogen.
From www.studypool.com
SOLUTION The nitrogen cycle review summary visual diagram explanation Combining Capacity Of Nitrogen Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. Nitrogen does not form stable. The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p x 3, therefore it could be expected to make 3. Valency is defined as the number of hydrogen atoms which combine directly or. Combining Capacity Of Nitrogen.
From www.alamy.com
Molecular Model of Nitrogen (N2) Molecule. Vector Illustration Stock Combining Capacity Of Nitrogen The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p x 3, therefore it could be expected to make 3. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The two nitrogen atoms are bonded together by a triple bond,.. Combining Capacity Of Nitrogen.
From pngtree.com
An Icon Showing The Element Nitrogen Group Symbol Periodic Vector Combining Capacity Of Nitrogen Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). The number of bonds that an atom can form as part of a compound is expressed by the. An active form of nitrogen, presumably containing free nitrogen atoms, can be created. Combining Capacity Of Nitrogen.
From www.sexizpix.com
Bohr Atomic Model Of A Nitrogen Atom Vector Illustration Sexiz Pix Combining Capacity Of Nitrogen Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The number of bonds that an atom can form as part of a compound is expressed by the.. Combining Capacity Of Nitrogen.
From www.alamy.com
Molecular model of Nitrogen N2 chemical molecule with one triple bond Combining Capacity Of Nitrogen The number of bonds that an atom can form as part of a compound is expressed by the. Nitrogen does not form stable. The two nitrogen atoms are bonded together by a triple bond,. Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. The simplest compound of nitrogen is. Combining Capacity Of Nitrogen.
From mavink.com
Draw And Label The Nitrogen Cycle Combining Capacity Of Nitrogen Nitrogen does not form stable. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. The number of bonds that an atom can form as part of a compound is expressed by the. The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p. Combining Capacity Of Nitrogen.
From www.alamy.com
Nitrogen cycle. The processes of the nitrogen cycle transform nitrogen Combining Capacity Of Nitrogen The combining capacity of an atom is known as its valency. Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. The number of bonds that an atom can form as part. Combining Capacity Of Nitrogen.
From www.biochemithon.in
Role of Nitrogen in Crops BioChemiThon BioChemiThon Combining Capacity Of Nitrogen The combining capacity of an atom is known as its valency. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The two nitrogen atoms are bonded together by a triple bond,. Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of. Combining Capacity Of Nitrogen.
From gardentabs.com
How Do Plants Use Nitrogen? Combining Capacity Of Nitrogen The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). Nitrogen does not form stable. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. An active form of nitrogen, presumably containing free nitrogen atoms, can be created. Combining Capacity Of Nitrogen.
From mavink.com
Ammonia Nitrogen Cycle Combining Capacity Of Nitrogen Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. The combining capacity of an atom is known as its valency. An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas. Combining Capacity Of Nitrogen.
From www.biochemithon.in
Role of Nitrogen in Crops BioChemiThon BioChemiThon Combining Capacity Of Nitrogen The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). An active form of nitrogen, presumably containing free nitrogen atoms, can be created by passage of nitrogen gas at low. The nitrogen has an electronic configuration 1sx2 2sx2 2px3 1 s x 2 2 s x 2 2 p x 3, therefore it could be expected to make 3. The combining. Combining Capacity Of Nitrogen.
From nitrogen-generators.com
Nitrogen Generators How It Works CGT Combining Capacity Of Nitrogen Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. The combining capacity of nitrogen, often referred to as its valency, is variable depending on the specific compound. The two nitrogen atoms are bonded together by a triple bond,. The number of bonds that an atom can form as part. Combining Capacity Of Nitrogen.
From www.adda247.com
Nitrogen Cycle Diagram, Steps, Drawing for Class 8 & 9 Combining Capacity Of Nitrogen The combining capacity of an atom is known as its valency. Valency is defined as the number of hydrogen atoms which combine directly or indirectly with one atom of an element. Nitrogen behaves chemically like nonmetals, nitrogen forms compounds in nine different oxidation states. The simplest compound of nitrogen is molecular nitrogen, \(\mathrm{n}_{2}\). The nitrogen has an electronic configuration 1sx2. Combining Capacity Of Nitrogen.